Alzheimer’s disease – Everything about dementia and its symptoms
Alzheimer’s disease – Everything about dementia and its symptoms : Alzheimer’s disease is one of the diseases of dementia, and also the most famous disease is memory impairment. This article will take an in-depth look at the pathology, diagnosis, and treatment of Alzheimer’s.
Alzheimer’s disease and dementia are not the same, although they are often used interchangeably. Dementia is a general term used for any severe mental retardation that interferes with the body’s health and daily life.
What is Alzheimer’s?

Alzheimer’s is a type of dementia that causes memory loss and impairs other critical mental functions. Alzheimer’s disease accounts for 60 to 80 percent of all dementia cases. It is a progressive disease; That is, the symptoms of Alzheimer’s gradually worsen over time. Alzheimer’s disease is the sixth leading cause of death in the United States. Patients with the disease survive an average of eight years after the onset of overt symptoms.
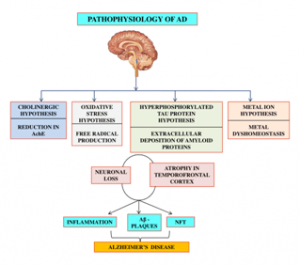
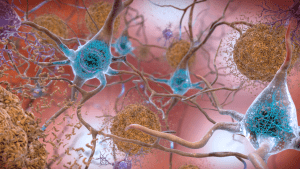
The human brain is made up of about 100 billion neurons, and Alzheimer’s kills millions of nerve cells and, in the advanced stages, leads to noticeable shrinkage of the brain. The main problem with Alzheimer’s disease is a disruption of communication between neurons in the brain; Neurons whose job is to process and transmit signals between different brain areas. This factor causes the death of brain cells and is related to amyloid and tau proteins’ production. The exact interaction between these proteins is largely unknown.
We know that amyloid accumulates in adhesive clusters called beta-amyloid plaques, and tao proteins accumulate inside dying cells as neurofibrillary tangles. Another critical issue is that beta-amyloid plaques are also found in the brains of healthy people. This indicates that amyloid and tao proteins do not give us definitive and accurate information about the disease. A new study suggests that chronic inflammation may play a role in causing the disease.
Inflammation is part of the immune system’s response to disease and occurs when white blood cells secrete certain chemicals to protect the body against a foreign body. However, prolonged inflammation and its chronicity can damage the body. In the brain, chronic tissue-damaging inflammation may be caused by an increase in “microglia.” In a healthy brain, these cells surround and destroy toxins. In Alzheimer’s patients, microglia cannot clear waste products such as amyloid-beta plaques and Tao masses from the cell. The body’s response to these conditions is to increase the number of microglia. An increase in these cells causes inflammation. So chronic inflammation damages explicitly brain cells and eventually causes them to die.
Types of dementia
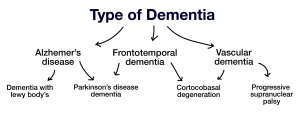
Alzheimer’s disease and the signs
After his death and autopsy, Dr. Alzheimer’s found that the brain’s outgrowth was much smaller than usual and that much fat had formed in his brain cells. The disease was discovered in 1906 by Dr. Alois Alzheimer and was named after him. He examined his brain and found out he had Alzheimer’s disease. Before Ms. Deter died, she showed many of the symptoms we know today as Alzheimer’s symptoms, including significant speech and cognitive impairments. Today, at least 4.5 million people in the United States have Alzheimer’s. That figure is five percent of Americans between the ages of 65 and 75. The prevalence of the disease among people 85 years and older is about 50%.
Alzheimer’s is a brain disease that is more common in the elderly but may occur in some people as young as 40. This disease is the most common cause of dementia and progressive; it worsens over time. Alzheimer’s disease changes the brain’s nerves; due to this change, the nerves lose their connection. People with Alzheimer’s usually do not live longer for about ten years after the first signs of the disease appear, but in some, the hope of survival is as high as 20 years.
Alzheimer’s youth
Premature Alzheimer’s or juvenile Alzheimer’s is an unusual form of dementia that affects people under 65. About 5 to 6 percent of people with Alzheimer’s show Alzheimer’s symptoms before 65. So if, for example, 4 million Americans have Alzheimer’s, about 200,000 to 240,000 of them have early-onset Alzheimer’s or Alzheimer’s. Most people with juvenile Alzheimer’s develop symptoms between the ages of 30 and 60.
Age is the most critical factor in developing Alzheimer’s; Other factors, such as heredity, diabetes, high blood pressure, brain damage, and poor nutrition, contribute to Alzheimer’s. As the disease progresses, short-term memory loss becomes so severe that the person’s daily activities are disrupted. Mild Alzheimer’s causes the person to need some help with daily activities. As the illness gets worse, help with daily chores such as cooking, bathing, and washing may become necessary. People with severe Alzheimer’s disease are entirely dependent on others for food and bathing and may not even use the bathroom without others’ help.
Frontal-temporal lobe dementia (FTD)
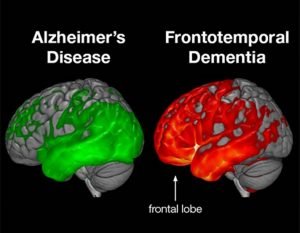
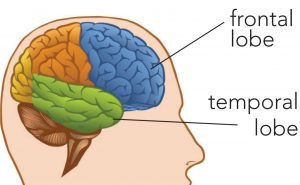
If your spouse has FTD, they have cell damage in areas of their brain responsible for controlling planning, judgment, emotion, talking, and movement. Like the globe, the human brain has different regions, such as continents, but interconnected, called brain lobes. There are two main lobes in the anterior and lateral parts: the frontal and parietal lobes. The frontal lobes are the center of attention, planning, judgment, and anything that separates us as civilized human beings from our predecessors. The main job of the parietal lobes is to help store memory as well as preserve our vocabulary.
The destruction of nerve cells in dementia of the frontal-temporal lobe dementia begins in these two main parts of the brain and spreads to other parts as the disease progresses. Temporal-temporal lobe dementia usually occurs in the 50s and 60s, but patients have also been observed in the 21s and 80s. About 60% of patients develop this type of dementia between 45 and 64, which is the age of professional activity, earning, and supporting a spouse and children. As a result, the financial burden of this type of dementia on families is heavier than in other cases of dementia. Behavioral and speech changes in this group of patients can severely impose a heavier burden on their caregivers’ Alzheimer’s disease. The most common types of forehead-temporal dementia are:
- Frontal variant: This type of dementia affects a person’s behavior and personality.
- Primary progressive aphasia
Aphasia, or more commonly aphasia, means difficulty communicating. This type of aphasia is divided into two groups: progressive verbal aphasia, impairs speaking, and semantic aphasia, which affects the ability to use and understand language. The cause of forehead-temporal dementia is unknown; Researchers believe that genetic mutations cause some types of it. The structure of brain cells in some people with the disease contains abnormal amounts of protein.
Vascular dementia
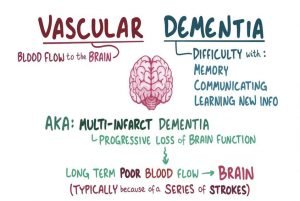
The most common type of dementia after Alzheimer’s is vascular dementia, which accounts for about 10 percent of all dementia patients and affects 1.4 million people. This type of dementia is caused by the blockage or rupture of cerebral arteries and the lack of oxygen and food to the brain’s nerve cells and their destruction. In this type of dementia, the disease symptoms may appear gradually, and any symptom may appear suddenly. For example, the patient may not be able to return to their previous level of mental function after a stroke, and in the event of another stroke, the patient’s performance may decline in the new context. Strokes do not always occur with noisy symptoms such as sudden paralysis. Multiple strokes may be asymptomatic not to cause noticeable symptoms to the patient and his companions.
Most patients with vascular dementia also have other illnesses and complications, including diabetes, high blood pressure, and high cholesterol, and many of them smoke. This type of dementia is more common in old age, but there are also cases under 60 years old. The incidence of disorders and complications varies in these patients. Impairment of judgment and decision-making power in routine daily planning may occur in addition to physical complications such as difficulty walking, paralysis, or numbness on one side of the face or half of the patient’s body. The location of vascular injuries, the severity of their occurrence, and the number of strokes directly affect the patient’s mental and physical ability. Vascular dementia symptoms may appear gradually or after a stroke or major surgery such as heart bypass or abdominal surgery.
Mixed dementia
About 10% of people with dementia develop more than one type of dementia called mixed dementia. The most common mixed dementia is the simultaneous presence of Alzheimer’s and vascular dementia in the patient.
Dementia with Louis Objects (DLB)
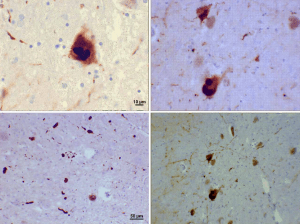
Luibadi is a protein deposit that is found microscopically in the brains of some people. They are named after the discoverer of these objects. If you know someone with DLB, it’s because of these objects’ deposition in a part of the brain called the cortex. According to the American Loubadi Organization website, this type of disease accounts for about 10% of all dementia patients, and the number of patients in the United States is 1.4 million. According to this organization, it is the second most common type of dementia in the United States. The incidence of Luibadi dementia and cerebrovascular dementia is very close, and there is a consensus on the second or third most common type of dementia in the United States.
This type of dementia is more common in old age, but there are also cases under 60 years old. Most of these patients have memory and thinking problems that are also present in Alzheimer’s. Brain tissue in people with the disease produces an abnormal protein called “lupus or lupus.” Luibadi is also found in the brains of people with Parkinson’s and Alzheimer’s. Of course, this protein is found in different parts of the brain of people with these diseases.
Louis Buddy’s dementia affects the ability to think, reason, and analyze information. A person’s ability to move, personality, and memory may also be affected. The incidence of this disease is related to age, and it usually appears in the sixties or seventies of life. Over time, physical complications and behavioral changes occur in them. Four main clinical features characterize Luibadi dementia. The first characteristic is that changes in the patient’s consciousness and mind vary during the day or week. So that in some cases, the patient is entirely typical, and in others, it is an entirely mental disorder. The second characteristic of this type of dementia is visual hallucinations when seeing people and animals.
In the realm of hallucinations, the patient may see and even speak clearly to relatives, objects, and animals that died years ago. The third characteristic of Loubadi dementia is symptoms similar to Parkinson’s disease in these patients. As a result, they may be misdiagnosed with Parkinson’s disease. The fourth characteristic of these patients is their high sensitivity to some drugs, especially antipsychotic drugs. These patients with shallow doses of these drugs may show side effects in other patients in high doses.
Rapidly progressive dementia
Most cases of dementia are slow and last for several years, but some pathogens can altogether disable a patient with a dementia sign within a few months. The list of these diseases is pervasive and includes infectious agents such as bovine insanity and HIV, metabolic factors such as hypothyroidism, or deficiency of vitamins and micronutrients.
Possible causes of Alzheimer’s disease
Amyloidosis proteins
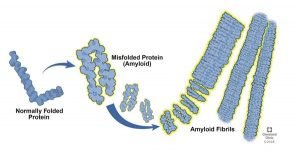
In Alzheimer’s disease, spherical protein structures are formed outside the nerve cells in some brain regions, and fibrous protein structures are formed in the neurons’ cell bodies. These protein structures, called amyloid bodies, are caused by specific changes in the protein of nerve cells and disturbances in the balance or changes in the amount or structure of the proteins of persuasion, apolipoprotein E, synuclein, and amyloid-beta peptide. One of the most important proteins involved in Alzheimer’s is called amyloid precursor protein (APP). This protein is present in the nervous system cells. It is involved in the attachment of cells to each other, cell contact, attachment to the extrinsic matrix, and the cytoskeleton—three types of proteolytic enzymes process APP protein. Alpha, beta, and gamma-secretase enzymes cleave APP protein into amino acids 678, 671, and 711, respectively.
By the effect of gamma and beta-secretase enzymes on APP protein, peptides called amyloid-beta 40 (containing 40 amino acids) and amyloid-beta-42 (containing 42 amino acids) are formed. Usually, the amount of these fragments in cells is small. They break down quickly, but if in the proteome of nerve cells this balance is disturbed and these fragments increase, spherical protein structures are formed, resulting in Alzheimer’s. Patients with Down syndrome (trisomy 21) have increased APP protein expression and Alzheimer’s-like symptoms, which may be due to an increase in the amount of amyloid-beta peptide 42; Because the APP protein gene is located on chromosome 21.
Multiple E4 genes
One study found that people with one copy of the E4 gene were three times more likely to develop Alzheimer’s disease than those without the gene.
Neuropathology
Near anatomical observation of a patient’s brain with Alzheimer’s disease with the naked eye shows dilatation of the cortical tortures and dilation of the brain’s ventricles. Classical microscopic findings also show the loss of neurons, the presence of aging plaques, and the loss of synapses.
Neural messengers
The most well-known neurotransmitters in Alzheimer’s disease are acetylcholine and norepinephrine, both of which appear to be less active in Alzheimer’s patients.
Aluminum poisoning

High levels of aluminum have been found in the brains of some Alzheimer’s patients. But this is not a significant factor.
Vascular dementia
The primary cause of vascular dementia is multiple cerebrovascular diseases that cause a pattern of dementia. The disease is more common in men and most often affects small and medium cerebral arteries.
Niemann Peak disease
Niemann Peak is a rare and inherited disease that affects fats (cholesterol and lipids) to metabolize within cells. These cells change function and die over time. Niemann Peak disease can affect the brain, nerves, liver, spleen, bone marrow, and lungs in severe cases. Also, Lewis’s body disease, Huntington’s disease, and Parkinson’s disease can lead to dementia.
Pathology of Alzheimer’s disease
Two decades before Alzheimer’s disease manifests itself, the brain begins to change slowly. The biggest of these changes is forming masses called amyloid plaques outside brain cells and forming protein filaments inside nerve cells called “NFTs.” These changes occur, especially in the learning and memory part of the brain. We do not know many things about filaments and plaques, but scientists are almost sure that they both play an important role in Alzheimer’s and causing problems in brain activity. Both amyloid-beta masses and these protein filaments interfere with transmitting messages from one neuron to another, resulting in proper brain function.
NFTs are nodular fibers that are likely to cause cell death in the long run. Usually, in the human brain, food passes through parallel and direct channels. The filaments prevent the proper transfer of food and block the way, thus destroying other cells that need fuel. As Alzheimer’s progresses, more and more brain cells die, and the level of neurotransmitters or neurotransmitters decreases. Neurotransmitters are substances that carry information to the brain from anywhere in the body. Simultaneously, the brain becomes inflamed, but doctors do not know if this inflammation plays an influential role in destroying plaques and filaments. The inflammation of the brain may be due to an all-out attack by the immune system on NFTs.
One of the recorded symptoms of Alzheimer’s is adhesive amyloid-beta protein, which forms around nerve cells. Most scientists believe that amyloid is responsible for the breakdown of information processing and cell death. However, researchers have pointed to another protein called tau, found inside brain cells, as the leading cause for more than a decade. A new study by imaging the brains of 10 people with mild Alzheimer’s has shown that tau deposition, not amyloid, is strongly linked to memory loss and dementia symptoms. Although this evidence does not address the amyloid-tau debate, it could lead researchers to new and more research into tau targeting therapy and better diagnostic tools.
Tau protein regulates neuronal growth, neuronal signal transmission, and cytoskeletal activity in the body called microtubules. But tau in the brains of people with Alzheimer’s has misaligned folds that lead to abnormal accumulations in the brain called neurofibrillary tangles. Scientists have long used an imaging technique called positron emission tomography (PET) to observe the deposition of radioactive-labeled amyloid-beta in the brains of people with Alzheimer’s. In addition to studies of brain tissue death, these studies have shown that people with Alzheimer’s have far more amyloid plaques in their brains than healthy people.
The problem is that about 30% of people have an amyloid-rich brain at autopsy without any signs of dementia. The question, then, is whether the tau protein, with its structural misalignment, may be the leading cause of neuronal damage and disease symptoms, or at least an essential factor. Until recently, the only way to test this hypothesis was to measure tau in the brain tissue after death or samples of a person’s cerebrospinal fluid (CSF) during life. But in the last few years, researchers have developed PET imaging so that the amount of tau in a living person’s brain can be accessed without any risk. Researchers used one of these tags in a new study and one tag attached to amyloid to examine both proteins’ deposition in 10 people with mild Alzheimer’s and 36 healthy adults.
Tau deposition was found mostly in the temporal lobe, where it is associated with memory in the brain and is associated with more disorders in memory test groups. This report is published in Science Translational Medicine. The same is not true of beta-amyloid. Although PET scans of amyloid-beta can lead to early detection of Alzheimer’s, tau is a better predictor when a person is in the early stages of mild Alzheimer’s without symptoms. The brain may also compensate for amyloid-beta damage, but the body cannot fight it when the tau begins to spread.
Researchers have found that the high tau levels found in patients’ CSFs are directly related to increased tau in the brain’s temporal lobe; An area in the brain involved in-memory processing. Therefore, tau in cerebrospinal fluid can be used as a diagnostic tool. Of course, this study was based only on a point in time in the participants’ brains, so it could not prove a link between increased tau and mental decline. Studies to track tau and amyloid in people’s brains are currently underway. These studies’ main goal is for researchers to predict which Alzheimer’s treatment is needed by each person based on what is happening in the brain.
The role of genetics in Alzheimer’s
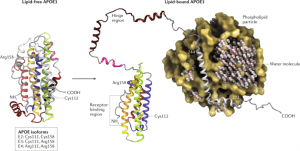
Unfortunately, research has shown that people who have a first-degree relative with Alzheimer’s are more likely to develop Alzheimer’s. If your parent has premature Alzheimer’s and inherited a gene mutation related to it, you can no longer prevent the disease. There are specific genes that influence or determine the likelihood of developing the disease. Determinant genes are genes that directly cause disease, and if a person carries these genes, they will certainly develop the disease like the gene that causes premature Alzheimer’s. Some genes increase the risk of getting the disease but do not cause the disease 100%. APOE-e4 is one of these genes present in 20 to 25% of people with Alzheimer’s.
Alzheimer’s disease is a complex multifactorial disease. About 10 percent of cases are inherited with autosomal dominant inheritance (AD). In hereditary Alzheimer’s disease (FAD), Alzheimer’s disease occurs early between 55 and 65. Of the approximately 90% of sporadic disease cases, 25 to 40% are associated with specific gene alleles. Examining the APP gene in family members whose individuals developed early-stage Alzheimer’s disease revealed various mutations in the gene that was not present in healthy individuals’ APP gene. Most of these mutations were malignant mutations in amino acids 716 and 717. Also, two simultaneous mutations in amino acids 671 and 670 were observed in many cases.
Mutation at 716 or 717 loci alters β and γ-secretase enzymes’ activity and increases the production of Aβ1-42 / 43). Simultaneous mutations at positions 671 and 670 also increase β-secretase activity and increase Aβ1–42 (43). Connection analysis was performed on family members with early-onset Alzheimer’s disease to obtain an area on chromosome 14 (14q24.3) associated with Alzheimer’s. The “RT-PCR” technique examined different mRNAs from this region, and by comparing mutations in the mRNA of patients with healthy individuals, the Alzheimer’s causative gene was identified. This gene encoded a protein called peridinin 1; This gene is represented by “PSEN1”.
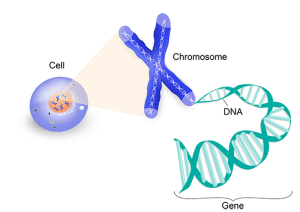
Similarly, another similar gene was identified on chromosome 1 in the 1q32-42 region, called “PSEN2”. Presenilin 1 and 2 proteins are about 67% similar and are membrane proteins. About 40% of premature familial Alzheimers are due to mutations in this gene; Mutations in this gene increase the amount of Aβ1–42 (43) (the mechanism of this action is not yet known). Other genes have been identified in connection with late familial Alzheimer’s. One of these loci is located on chromosome 19 in the 19q13.2 region, where the APOE gene encodes apolipoprotein E. This gene has three alleles, and further studies have shown that the APOE-e4 allele is associated with Alzheimer’s disease. Of course, this allele’s presence as homozygous or heterozygous in a person is not necessarily associated with Alzheimer’s, and this allele only increases a person’s chances of developing Alzheimer’s.
This allele is considered a risk factor for Alzheimer’s; Other factors, such as environmental and physiological factors, in addition to the APOE-e4 allele, are required for the development of Alzheimer’s (multifactorial). Also, alleles of the A2M gene on chromosome 12 in region 12p13.3 and LRP1 on chromosome 12 in region 12q13-14 are considered risk factors for Alzheimer’s disease. They encode alpha-2-macroglobulin (A2M) and LDL1 receptor-dependent proteins (LRP1), respectively. LRP1 binds to A2M, APP, and APOE, facilitating APP degradation. In the mutation in these two proteins, APP degradation is not performed quickly, and the amount of amyloidogenic proteins increases due to imbalance.
Diagnosis of Alzheimer’s disease
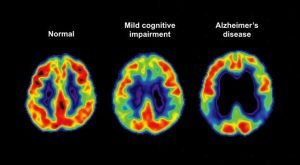
One of the problems with Alzheimer’s disease is that there is no measurable and accurate way to measure these proteins, which begin to increase in the early stages of the disease. We can diagnose Alzheimer’s only after a patient dies and by testing their brain tissue. At present, there is no test to determine if a person has Alzheimer’s or is at risk for the disease. Diagnosis of Alzheimer’s requires careful medical evaluations, including physical and neurological examinations and mental health tests. While there is no way to prevent or eliminate Alzheimer’s disease progression, early detection can increase the likelihood that medications will work.
Detection with nanopores
By bypassing proteins through nanopores, researchers have been able to obtain information about their structure. Because the passage of different proteins has different effects, this method can also diagnose diseases rapidly. Researchers at the University of Pennsylvania have taken great strides in finding a way to determine the order of DNA bases as they pass through a nanoscale hole. Also, in this study, they found that this method could learn more about the proteins’ structure. The methods currently available for this require a great deal of work and the use of large amounts of protein. Changes must also be made to proteins that limit these models’ efficiency in understanding proteins’ behavior in their natural and real state.
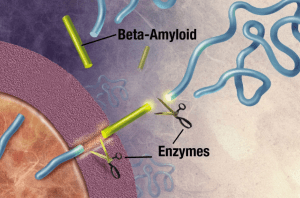
The translocation method, introduced by researchers at the University of Pennsylvania, makes it possible to study individual proteins without modifying them. In this method, by examining individual samples, it is possible to diagnose diseases and research them. This method is used to detect the order of genes with nanopores’ help, aiming to identify the bases (adenine, thiamine, guanine, cytosine) in a strand of DNA by examining the sizing orifice they cover as they pass through the nanoscale cavity. Different effects on the residue of this passage allow different amounts of ionic liquids to pass through. Electronic devices around the cavity measure the changes in ion current, and the ups and downs of this signal can be related to each of the bases.
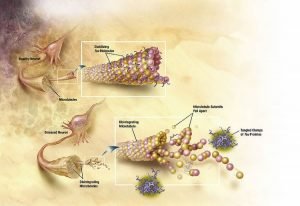
The enzyme cuts the APP molecule into small pieces. Amyloid-beta fragments play a vital role in the formation of Alzheimer’s plaques.
Drendick and colleagues conducted experiments to investigate this method’s effects on other biological molecules and nanoscale structures. In collaboration with the Sawn Group, they performed experiments on molecules that required more precision. “It says,
We want to study; Proteins that are much smaller than DNA strands and are very difficult to work with. We are interested in having a lot of information about a protein structure, such as whether it exists as a monomer or combines with another protein and is a dimer or a community of many proteins and exists as an oligomer.
The researchers poured different monomer and dimer ratios into an ionic fluid and passed it through the cavities. Monomers and dimers block different numbers of ions. So when they pass through the hole, different currents are observed. Many researchers have observed long strands of peptides (a group of amino acids) and proteins in Alzheimer’s and Parkinson’s diseases. Still, there is ample evidence that these strands are associated with post-disease, and the leading cause of the disease is a set of smaller proteins. But it is tough to discern the nature and size of these collections in the current situation.
Gene markers
Scientists at King’s College London have developed a gene marker that can be used to predict the onset of diseases such as Alzheimer’s before it develops. This study seeks to define a set of genes associated with healthy aging at age 65. Such molecular profiles can be useful in diagnosing people at early risk of age-related diseases. This success provides the first strong molecular marker of biological age in humans and could change the way age is used for medical decisions.
This includes identifying people who are most likely to be at risk for Alzheimer’s disease. The researchers analyzed RNA from 65-year-old subjects and used the data to produce a marker consisting of 150 RNA genes that indicate healthy aging. This marker is a reliable predictor of the risk of age-related disease when studying organs such as human muscle, brain, and skin. Using this marker, scientists generated a healthy age gene score and used it to test and compare different people’s profiles.
Saliva test
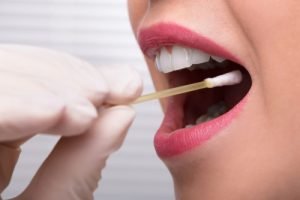
Many diagnostic methods for Alzheimer’s can be invasive and costly, reinforcing the motivation to look for more straightforward and cheaper methods. For this reason, researchers at the University of Alberta in Canada analyzed saliva samples from 22 Alzheimer’s patients, 25 patients with mild cognitive impairment (MCI), and 35 patients with normal cognitive function using liquid chromatographic-mass spectrometry (LCMS). The researchers identified more pronounced compounds in Alzheimer’s and MCI patients’ saliva and distinguished them from healthy individuals. Further studies showed that higher levels of specific substances in participants’ saliva were closely associated with lower cognitive function. For example, higher levels of a specific compound in the saliva of participants with Alzheimer’s were associated with slower information processing speeds. The team believes their findings promise more non-invasive and cheaper diagnostic methods for Alzheimer’s.
Biomarker test
A biomarker is something that can be detected when measured. The two proteins, amyloid-beta, and tao, found in the brains of people with Alzheimer’s, can be measured in the fluid that surrounds the brain and spinal cord (cerebrospinal fluid). This fluid is used to show the abnormal progression of amyloid-beta proteins, which make up plaques, and tau proteins, which make up filaments. These proteins can diagnose Alzheimer’s disease as another cause of dementia and help identify people with the disease before the severe mental decline. They can support the diagnosis of Alzheimer’s but have not yet been routinely used for diagnosis.
Brain imaging
Researchers are studying imaging techniques, such as MRI and positron emission tomography, used with a radio tracker. Radio trackers are charged particles that glow and show infected areas in brain images; For example, by binding to proteins associated with Alzheimer’s, amyloid, and tau. However, having amyloid plaque in the brain does not mean that you have dementia.
blood test
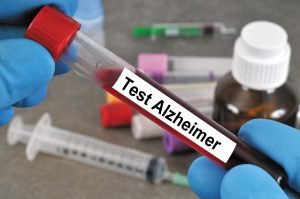
This test identifies people whose brains have high levels of amyloid beta. Researchers hope that drug development organizations will use this test to attract people with Alzheimer’s before severe injuries to their brains and conduct clinical trials on them, making the test results more reliable. To date, the only way to assess the amount of amyloid-beta in the brain (without autopsy) was by positron emission tomography. Also, the number of proteins was determined directly from the cerebrospinal fluid (CSF). Although both methods were effective, they were both costly and challenging to implement.
To measure the amount of amyloid-beta fragments in a blood sample or the amount of larger protein from which the amyloid-beta fragments originated, Dr. Yangisawa and colleagues combined two methods: immunoprecipitation and mass spectrometry. Their findings were entirely consistent with brain imaging and cerebrospinal fluid analysis in 121 Japanese and 252 Australians. Most of these people were in their 60s and 90s. Some were healthy, some had mild cognitive impairments, and some were diagnosed with Alzheimer’s.
Alzheimer’s treatment

More than 6 million Americans have Alzheimer’s disease or dementia. Even as the average age of the population increases, this number is expected to increase dramatically. For decades, doctors worldwide have been looking for ways to strengthen the brain and prevent Alzheimer’s in old age. Researchers have tried a wide range of therapies, including exercise, brain games, and medication. Systematic studies published recently on the evidence of Alzheimer’s prevention and treatment do not bode well. For the past 25 years, the focus of treatment has been on the amyloid cascade hypothesis. It is hypothesized that the formation of plaques of amyloid-beta (Aβ) peptides in the brain causes neuronal death and cognitive impairment.
However, evidence linking Alzheimer’s disease to these plaques was ineffective in targeting the peptide and reversing cognitive decline. Researchers in a review article published in the journal suggests a complex set of changes at the molecular, circuit, and network level and the increase in Aβ plaques that may trigger a series of disease-causing phenomena that are progressing. Alzheimer’s is involved. Importantly, these changes are not easily reversible by controlling the Aβ level. It has been shown that a small amount of improvement can be achieved by explicitly targeting the neurotransmitter system in the brain; In fact, it has changed the activity of the network and brain circuit and can reduce and eliminate cognitive decline.
Small-scale human studies have also shown that deep brain stimulation has had promising effects on improving cognition. Careful examination of areas of the brain damaged by Aβ plaques and other damage that contribute to the progression of Alzheimer’s may help researchers identify the early signs of disease progression. In this way, by targeting the injury source and the premature reduction of Aβ levels, researchers can target specific circuits or areas of the brain and improve cognitive function. It also allows clinicians to identify people at high risk for Alzheimer’s before the onset of cognitive decline symptoms and prevent disease progression.
Medication
Suppose dementia is caused by factors such as trauma, side effects of medications, and vitamin deficiencies. In that case, it may be possible to prevent damage to the brain or the spread of damage to brain tissue. The symptoms of Alzheimer’s disease can be reduced with the help of some medications. There are four drugs approved in the United States called cholinesterase inhibitors (acetylcholinesterase): donepezil, galantamine, rivastigmine, and tacrine. Another class of drugs is called memantine, which works against NMDA receptors in the brain. These drugs may be used alone or in combination with cholinesterase inhibitors. Cholinesterase inhibitors can also help improve the behavioral characteristics of a person with Parkinson’s disease. (Everything About Parkinson’s: Symptoms, Prevention, And Treatment)
Remember never to take these medications arbitrarily, and be sure to consult your doctor before taking any medication. Researchers at the Institute of Human Microbiology (HMI) and the Tetz Laboratories have discovered thousands of prion-like domains in human viruses that have set new goals for developing new antiviral drugs. Prions are contagious proteins that can reproduce spontaneously due to their rich β-plate structure, leading to the accumulation of erroneously folded proteins and neurotoxic effects in the brain, known to have neurodegenerative consequences. However, the exact cause of human prion formation is not yet known. The discovery of prions in viral proteins reveals an unknown pathway for diseases associated with misfolded proteins, including Alzheimer’s and Parkinson’s disease, ataxia, and amyotrophic lateral sclerosis. Previous research has shown that viruses are involved in the development of these diseases. The findings of HMI researchers show, for the first time, that the wrong folding of proteins is the unknown pathway of these prions to infect humans. Casey Maguire, Assistant Professor of Neurology at Massachusetts General Hospital, considers the discovery of prion-like domains in viral structures to be important in virology. He says:
These researchers have found new targets that could produce new antiviral drugs, which could have significant implications for treating and combatting all diseases.
Cellulotherapy
A new study suggests that implanting a specific type of neuron inside the brain may restore cognitive function in patients diagnosed with Alzheimer’s. Like a large orchestra, the brain relies on the perfect harmony of many elements to function correctly, and if one of them goes out of harmony, it affects the whole set. For example, in Alzheimer’s disease, damage to specific neurons can alter brain waves’ rhythm and lead to cognitive function loss. Researchers at the Gladstone Institute in the United States say a type of neuron called an inhibitory internode is especially important for controlling brain rhythms.
In this study, scientists discovered the therapeutic and healing benefits of these interneurons and their implantation in an Alzheimer’s mouse model’s brain. Interneurons control complex networks between neurons, allowing them to send signals to each other in a coordinated manner. Interneurons create brain rhythms to guide stimulus neurons both during activity and at rest. An imbalance between these types of neurons causes disorders and is seen in many neurological and psychological disorders such as Alzheimer’s disease, epilepsy, schizophrenia, and autism. “The Gladstone Institute,” said George Gallup.
These fused interneurons can be well integrated into new brain tissues, and each interneuron will be able to control thousands of excitatory neurons. These properties make interneurons a promising therapeutic target for cognitive disorders associated with brain rhythm abnormalities and epileptic activity.
Initially, scientists overcame a critical challenge because they did not positively affect transplanted regular neurons.
Because Alzheimer’s disease creates a toxic environment inside the brain, the researchers then genetically amplified the activity of inhibitory interneurons with a protein called Nav1.1. They found that enhanced function neurons overcame the toxic environment of the disease and restored brain function. The new study is published in the journal CELL.
Gene Therapy

For the first time, new research has shown how a known genetic risk factor for Alzheimer’s disease affects human brain cells. They have also been able to modify the gene and eliminate its destructive effects. The apolipoprotein gene (APOE) in the development and progression of Alzheimer’s disease has been studied. For example, researchers know that having an APOE4 gene variable increases the risk of Alzheimer’s by two to four times. Having two copies of this genetic variation is also associated with a 12-fold increase in infection risk. APOE, in combination with fats, produces apolipoprotein. Apolipoprotein helps transport and regulate cholesterol levels in the bloodstream.
But the E4 version of this gene is destructive to the brain. Several studies have shown that this genetic variable increases the risk of developing amyloid-beta and tao toxins. The researchers found that the APOE4 protein in the human brain has a pathogenic composition. That is, it has an abnormal shape in the brain that prevents the protein from working correctly. This abnormal form leads to several disease-causing problems. APOE4-expressing neurons have higher levels of tau phosphorylation. Also, the production of amyloid-beta peptides is higher in these people, which leads to the destruction of gabaergic neurons. High levels of tao phosphorylation have nothing to do with the increased production of amyloid-beta peptides. The study is published in the journal Nature.
MIND regime

Dementia and decreased brain function are some of the issues that affect many people with age. The brain, like other organs, needs proper nutrition to function correctly. The MIND regimen is designed to prevent dementia as well as prevent the decline of brain function. This diet combines the Mediterranean diet and the DASH diet (diet to stop high blood pressure), designed to create a unique diet pattern to maintain brain health. This type of diet focuses on the mind and brainpower. The Mediterranean diet is based on local and regional foods. It focuses more on fruits, vegetables, legumes, lentils, and whole grains, limiting red meat consumption, dairy products, sweets, and fatty foods.
Olive oil contains unsaturated fatty acids and can have good heart health benefits. For this reason, this oil has a special place in the MIND diet. Research on the MIND regimen has failed to show precisely how it works. However, the scientists who designed this diet believe that the MIND diet can reduce oxidative stress and reduce inflammation. Oxidative stress occurred when unstable molecules called free radicals accumulate in large quantities in the body. This condition often leads to cell damage.
The brain is also particularly vulnerable to this condition. Inflammation is the body’s natural response to injury and infection. But if not properly regulated, it can be harmful and contribute to many chronic diseases. Oxidative stress and inflammation, in general, can be harmful to the brain. These two factors have been a significant component of some treatments for Alzheimer’s disease in recent years. Following the Mediterranean and DASH diets is involved in reducing oxidative stress and inflammation. Since the MIND diet is a combination of these two diets, it can be concluded that it is likely to have antioxidant and anti-inflammatory effects. The antioxidants in berries and vitamin E in olive oil, leafy vegetables, and nuts can help improve brain function by protecting against oxidative stress.
The omega-3 fatty acids in fish are also known to reduce inflammation in the brain positively; They also slow down brain function loss. Researchers also believe that the MIND diet may have beneficial effects on the brain by reducing amyloid-beta proteins. Animal and laboratory studies have shown that antioxidants and vitamins in foods recommended in the MIND diet play a role in preventing amyloid-beta plaques in the brain. The MIND diet also restricts eating foods high in saturated fats and trans fats. Research has shown that these foods increase the levels of amyloid-beta protein in the brains of mice. Researchers have concluded from human studies that consuming these fats can double the risk of Alzheimer’s disease.


